Cancer cells are capable of migrating through the body for multiple reasons but determining which cells are moving and why can be difficult. Now, Purdue University researchers have reverse-engineered a cellular signal processing system and used it like a logic gate – a simple computer – to better understand what causes specific cells to migrate.
The research team built a microfluidic device that mimicked the environment of cancer cells. The device was designed with two chambers separated by an insulating wall so that different chemical signals could be applied in each chamber. The team then used these signals as inputs to the logic gate, which allowed them to observe how the cancer cells behaved when exposed to different combinations of signals.
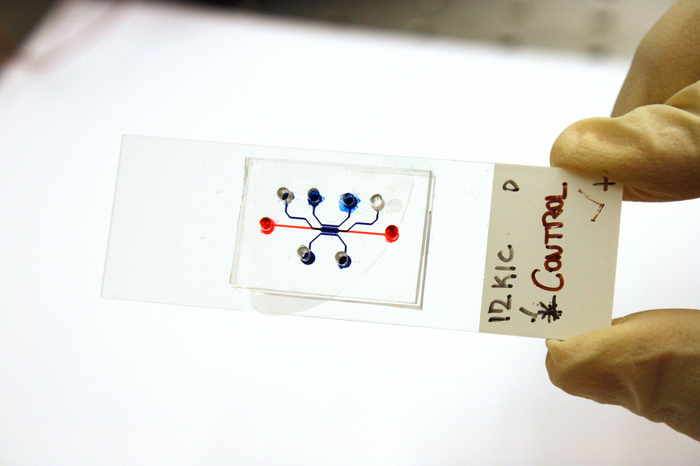
Purdue University photo/Jared Pike
The team found that when exposed to certain combinations of signals, some cancer cells traveled faster than others. By analyzing which combination of signals caused this behavior, they were able to determine what triggers it. They also observed that when exposed to high levels of glucose and oxygen or low levels of oxygen and glucose, some cancer cells moved at faster speeds than others. This suggests that cancer cell migration is not just driven by nutrients alone; it is also affected by other factors such as oxygen levels and glucose concentration in the environment.
In addition, the researchers discovered that when exposed to certain combinations of signals, some cancer cells did not move at all, while others moved very slowly. This suggests that there may be a threshold level beyond which cancer cell migration is inhibited or stopped altogether. This could lead to new treatments for cancers where cell migration is an issue.
In summary, Purdue University researchers have developed a cellular signal processing system modeled after a logic gate – a simple computer – to better understand what causes specific cancer cells to migrate through their environment. By experimenting with different combinations of input signals and observing how the cancer cells responded, they were able to identify certain triggers for cell migration as well as thresholds beyond which cell migration was inhibited or stopped altogether. This breakthrough provides insight into how we can control and manipulate cancer cell behavior with targeted therapies in order to treat cancers where cell migration is an issue.