
Omniphobic surfaces are those that can repel pretty much any kind of liquid. These kinds of special surfaces could potentially be very useful when it comes to reducing drag in a ship’s hull or producing a stain resistance layer to protect against harmful chemicals.
The problem is that most of the omniphobic surfaces developed to date quickly lose their ability to shed liquids as soon as condensation appears. To get around that issue, researchers at MIT produced a new kind of surface design that significantly reduces the effects of condensation, with just a slight sacrifice in its efficiency.
Creating a surface that’s able to shed pretty much any liquid takes a special kind of texture that creates tiny microscopic air pockets to separate the liquid from the surface. “Many liquids are perfectly wetting, meaning the liquid completely spreads out,” explains graduate student and professor of mechanical engineering, Kyle Wilke. This includes a lot of the refrigerants used in household appliances, hydrocarbons such as those contained in lubricants and fuels, and many kinds of alcohol.
While there are various fabrication methods around, they’re all far from perfect. The problem is that they’re working with surface features measuring in a less than a millionth of a meter that is extremely fragile. If just one part of the surface is damaged it can ruin the whole process.
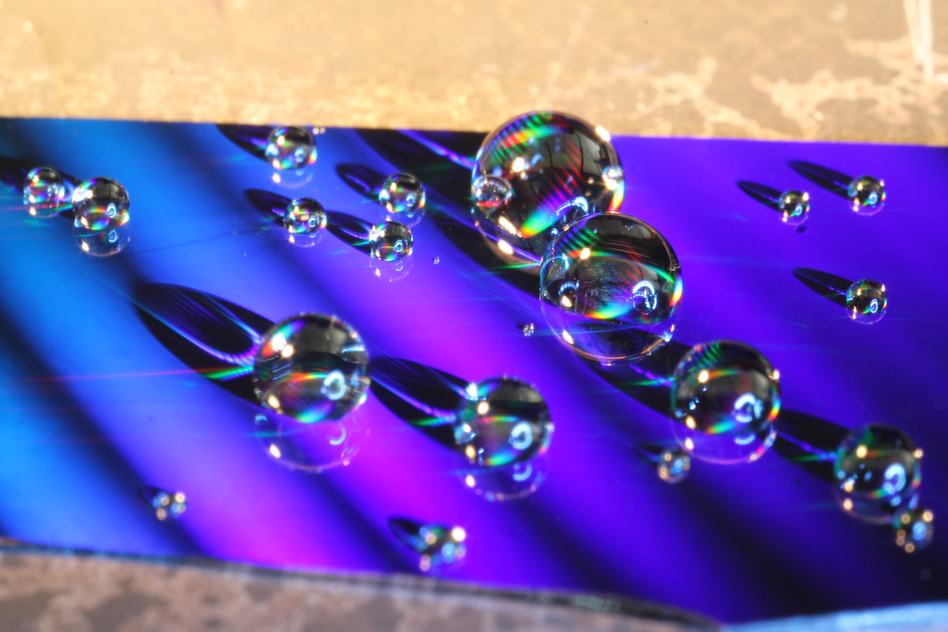
Images: Kyle Wilke
“We wanted a structure that one defect wouldn’t destroy,” said Wilke. Eventually, they discovered a geometry that fit the bill, and that was to have microscopic air pockets that are disconnected from the surface, making the chance of spreading between them less likely. The key element of the architecture is that of the ridges that help the surface achieve its resistance to both damage and condensation. And it’s the way in which these ridges are connected that prevents penetration of the liquid through the surface.
The way the ridges are made is via a multistep process using a standatd microchip manufacturing system. First, the spaces between the ridges are etched away, then the edges of the pillars are coated, before finally etching an indentation in the ridges’ sides.
Wilke says the only real reason omniphobic surfaces are not used more often, is due to the limitations of the current technology. But this could change as they improve on their durability and resistance to condensation. The system still has a way to go before it’s finished, but potentially it could be used to improve resistance to the build-up of ice or to make self-cleaning surfaces among other things.
Omniphobic surfaces are also good for providing protection against corrosion by limiting contact between the surface and any corrosive liquid it comes into contact with. And, because of the way in which the surface is so precisely designed, the new technique can be used for “tailoring how a surface interacts with liquids, such as for tailoring the heat transfer for thermal management in high-performance devices,” says Wilke.
One of the main limitations of these surfaces is that once the liquid gets into the voids, the entire surface becomes wetted. However, this new approach fixes that issue. Chang-Jin Kim is a professor of mechanical and aerospace engineering at the University of California. And, although Kim was not involved in the study, commented that this approach “can potentially make some of the omniphobic surfaces useful and practical for some important applications.”
