A recent study’s been carried out by Rice University researchers that take a deeper look into hydrogen-producing atom-thick catalysts that to see just where it’s coming from. The researchers are hopeful that the results from the study will help in the development of 2-D materials which could be used in energy applications such as fuel cells.
As part of the research, scientists Jun Lou and colleagues at Los Alamos National Laboratory developed a technique that allowed them to peer through windows created by an electron beam in order to measure the catalytic activity of molybdenum disulfide – the 2-D material that shows potential for being used in applications using electrocatalysis to separate hydrogen from water.
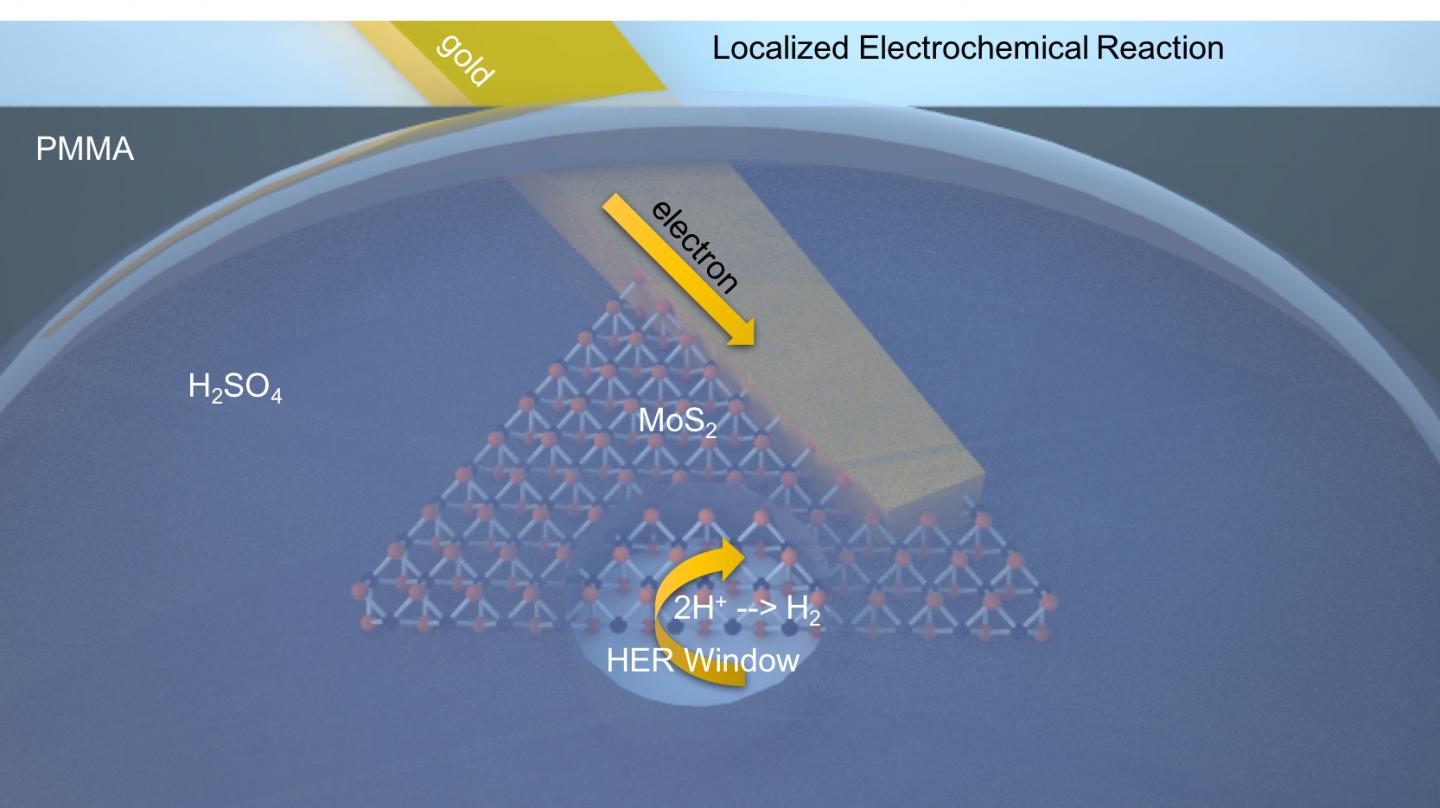
Results from the initial tests proved that the sheet’s edge is where most of the production is coming from. “We’re using this new technology to identify the active sites that have been long-predicted by theory,” said Lou. “There was some indirect proof that the edge sites are always more active than the basal planes, but now we have direct proof.”
The new research will help pave the way for faster screening of possible hydrogen evolution reaction candidates. “The majority of the material is on the surface, and you want that to be an active catalyst, rather than just the edge,” confirmed Lou. “If the reaction only happens at the edge, you lose the benefit of having all the surface area provided by a 2-D geometry.”
Researchers went on to test molybdenum disulfide flakes with the 1T prime (or distorted octahedral) and 2H (trigonal prismatic). “They’re basically the same material with the same chemical composition, but the positions of their atoms are different,” confirmed Lou. “1T prime is metallic, and 2H is a semiconductor.”
Prior studies showed that 1T prime was catalytic across its entire surface area, but the Rice study showed how the 1T prime edge is much more active than the basal plane. This was a complete discovery. Lead author Jing Zhang, a postdoctoral researcher, used an electron beam evaporation technique in which to deposit electrodes to individual flakes that had been made via chemical vapor deposition. Next, an insulating layer of poly (methyl methacrylate) was added to the mix and a pattern of windows burned into the material via e-beam lithography. In doing this, the researchers were able to then probe both the basal planes and edges of the 2-D material at submicron resolution.
Having 16 probes packed into an inch-square chip enables data to be retrieved from multiple sites on a single or multiple flakes. Being able to carry out rapid testing will help researchers come up with more efficient materials in which to maximize the catalytic activity of the basal planes.
More News to Read
- International Team Proposes New Quantum Technology to Help Transfer Data from Light to Matter
- Researchers Discover Way of Producing High Energy Photon Beams
- Early Breast Cancer Detection Made Possible Through Artificial Intelligence
- Swiss Researchers Create Batteries Out of Waste Graphite
- Help Comes in the Form of Biosensors for Cystic Fibrosis Patients

