There are three laws of thermodynamics. The first is referred to as the Law of Conservation and Energy and states that energy can neither be made or destroyed in an isolated system and the second law states how the entropy of an isolated system will always increase. Physicists worldwide have accepted these two laws quite easily, but the third has not been welcomed quite so much.
The third law of thermodynamics states that the entropy of a system approaches a constant value once the temperature nears absolute zero and is a law that’s been questioned continually by various scientists. Now, researchers from the University College London (UCL) have proved in their paper that absolute zero can’t be reached in a system where entropy is unable to reach zero. Lluis Masanes is a member of the research team and said, “We show that you can’t cool a system to absolute zero with a finite amount of resources and we went a step further…We then conclude that it is impossible to cool a system to absolute zero in a finite time, and we established a relation between time and the lowest possible temperature. It’s the speed of cooling.”
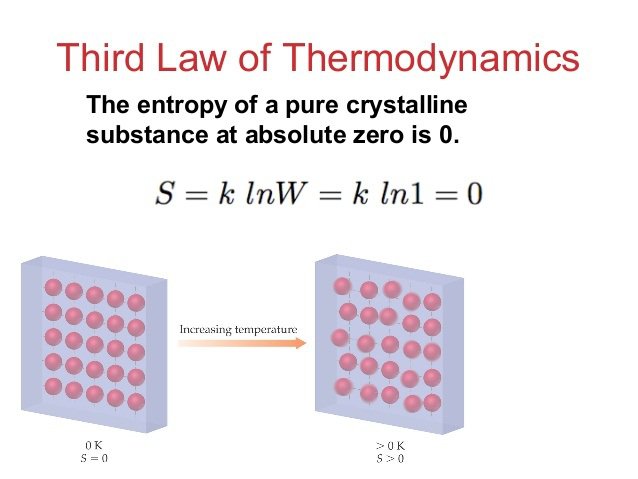
The team is hoping that their research will aid others in their studies and also help push forward the idea of quantum computing. By understanding the rate at which we can cool things may help people understand better the law of thermodynamics and thus make further developments in the world of quantum physics.
More News to Read
- How Might Arsenic Molecules be used to ‘Fish Out’ Toxic Elements from Radioactive Nuclear…
- Now We Can Get An Even lose Look at Black Holes Thanks to CHIRP
- Are Robots Killing the American Dream?
- ALMA Captures Dying Red Giant Star in Its Final Moments
- US Manufacturing Vs. China Manufacturing: Who Has the Lead?