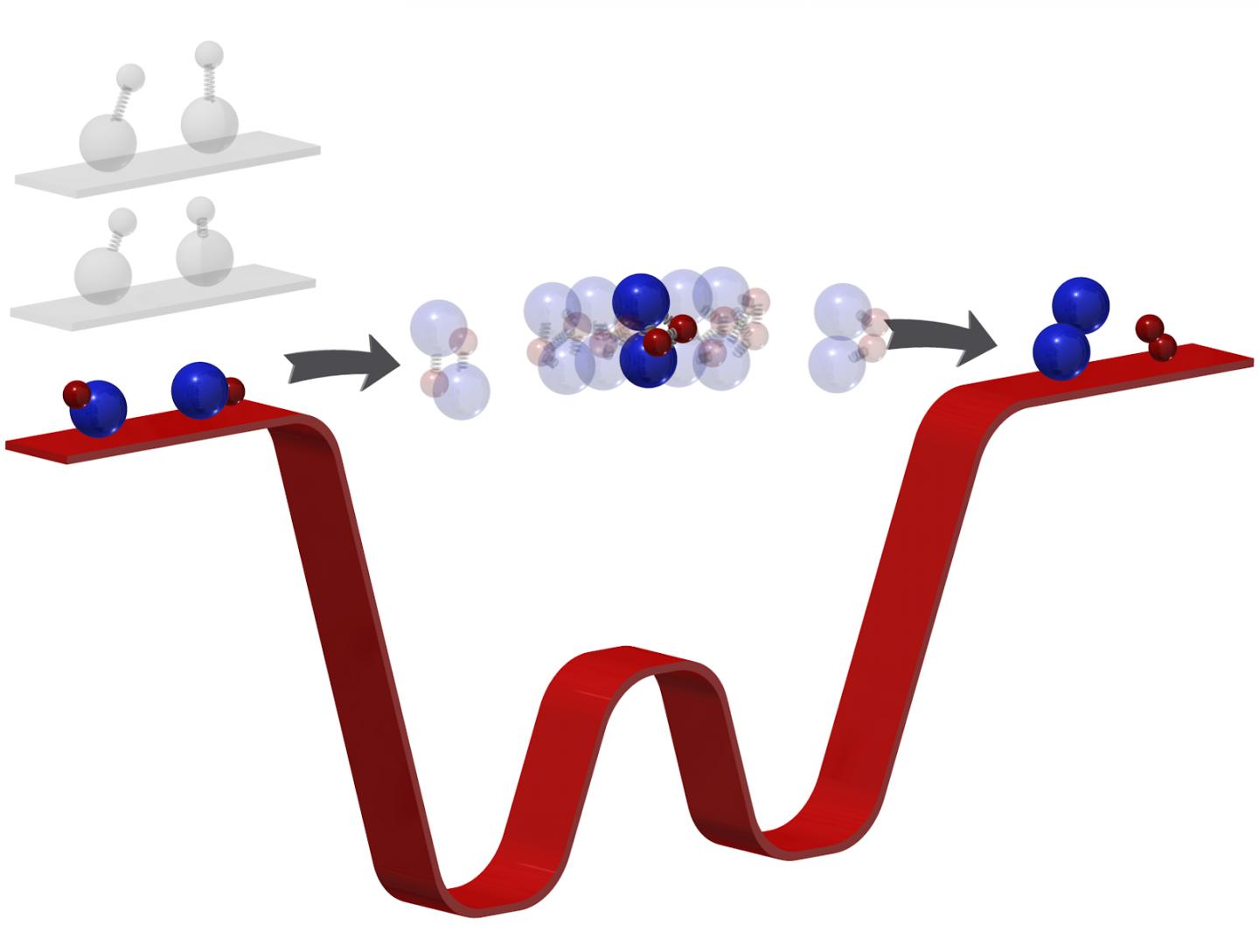
A world-first has been recorded in the world of ultracold chemistry as Professor Kang-Kuen Ni creates the coldest chemical reaction ever. By forcing together two ultracold molecules, Ni managed to break and form the coldest bonds we’ve ever seen in the history of ultracold chemistry.
Back in 2014, Ni and a colleague began constructing a new kind of apparatus that would enable the lowest ever temperature chemical reactions to take place. The problem was they were unsure their plans would work. But, they did. And, not only did they achieve performing the coldest reaction ever, they also managed to see it in full action.
Capturing a chemical reaction is no easy feat. But, in temperatures of -500 nanokelvin molecules much slower, enabling Ni and her team to see exactly what was happening when two molecules meet to form two new ones. Understanding how chemical reactions work at this level could help the world evolve massively.
CREDIT Ming-Guang Hu
In Ni’s previous studies around ultracold reactions, she was only ever able to see the start of the reactions. “There was no direct measurement of what actually happened in these chemical reactions, “she said. But, now, it’s a whole different story. “Because the molecules are so cold,” says Ni, “now we kind of have a bottleneck effect.” And it’s this bottleneck effect that allows them to see what’s going on during the chemical reaction as bonds break and form.
Next on the horizon for Ni and her team is to see how else they can use the ultracold test bed to learn. One thing it could be used for is to excite the reactants before the reaction to see what impact their heightened energy had. Or, they could even try and influence it as the reaction takes place by nudging one or more of the molecules.
“With our controllability, this time window is long enough, we can probe,” said Ming-Guang Hu, a postdoctoral scholar in the Ni lab and first author on the paper. “Now, with this apparatus, we can think about this.”

