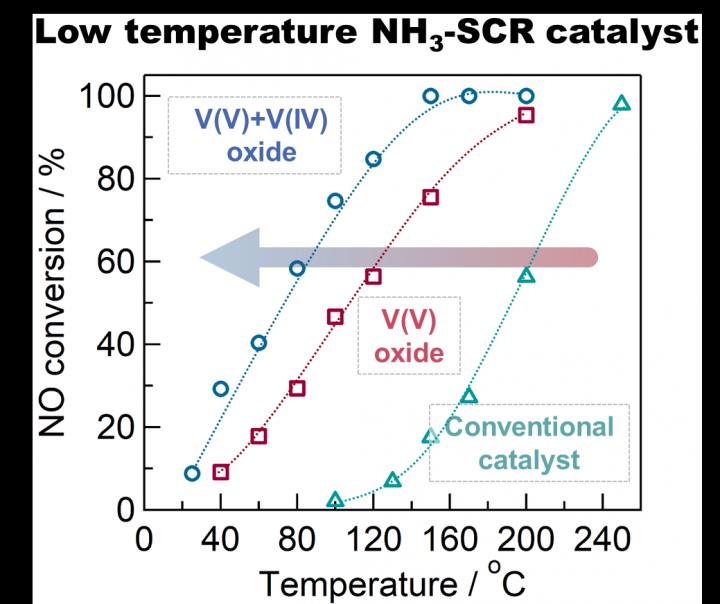
Replacing vanadium oxides supported on titanium oxide with bulk vanadium oxide enables the removal of NOx (nitrogen oxide) gas from industrial exhausts at a much lower temperature and higher efficiency. And now, a team of scientists from Tokyo Metropolitan University has used that knowledge to develop a catalyst in which to do this.
Nitrogen oxides (NOx) are very common pollutants found in the air which are created by burning coal, gas, and other fossil fuels. They are a big contributor to both smog and acid rain which is the main reason why the removal of them from vehicle and factory emissions is so important.
CREDIT Tokyo Metropolitan University
One way in which to remove nitrogen oxide is through the use of ammonia in a process called selective catalytic reduction (SCR). In this process the NOx is reduced to nitrogen and water, rendering it harmless. Usually, these catalysts require a high temperature in which to work, often between 200 to 400 degrees Celsius. The problem with this is that not only is there a higher chance of units becoming damaged by the high heat, but also an accumulation of ammonium sulfates.
However, a team of researchers from Tokyo Metropolitan, led by Yusuke Inomata and Toru Murayama has developed a catalyst that will work at lower temperatures based on bulk vanadium oxides. Using a synthesized mixture of vanadium and vanadium oxides, the team found that the catalyst still worked at temperatures even lower than 100 degrees Celsius. And, what’s more, is that it converted NOx into harmless nitrogen 10 times faster than traditional catalysts.

