There’s all kind of chemistry that happens in nature. Water, for example, is continuously broken down into its oxygen, electrons, photons, and brought back again as a way for plants and animals to store and use energy. It’s those kinds of natural processes and special enzymes that could help researchers develop new pathways to meet the growing energy demands of today’s population.
Over at Kyushu University, researchers have developed a single catalyst that can mimic two natural energy processes. Not only does it act as a fuel cell that consumes hydrogen to release energy, but it also acts as the photosynthetic system that uses solar energy in which to produce oxygen.
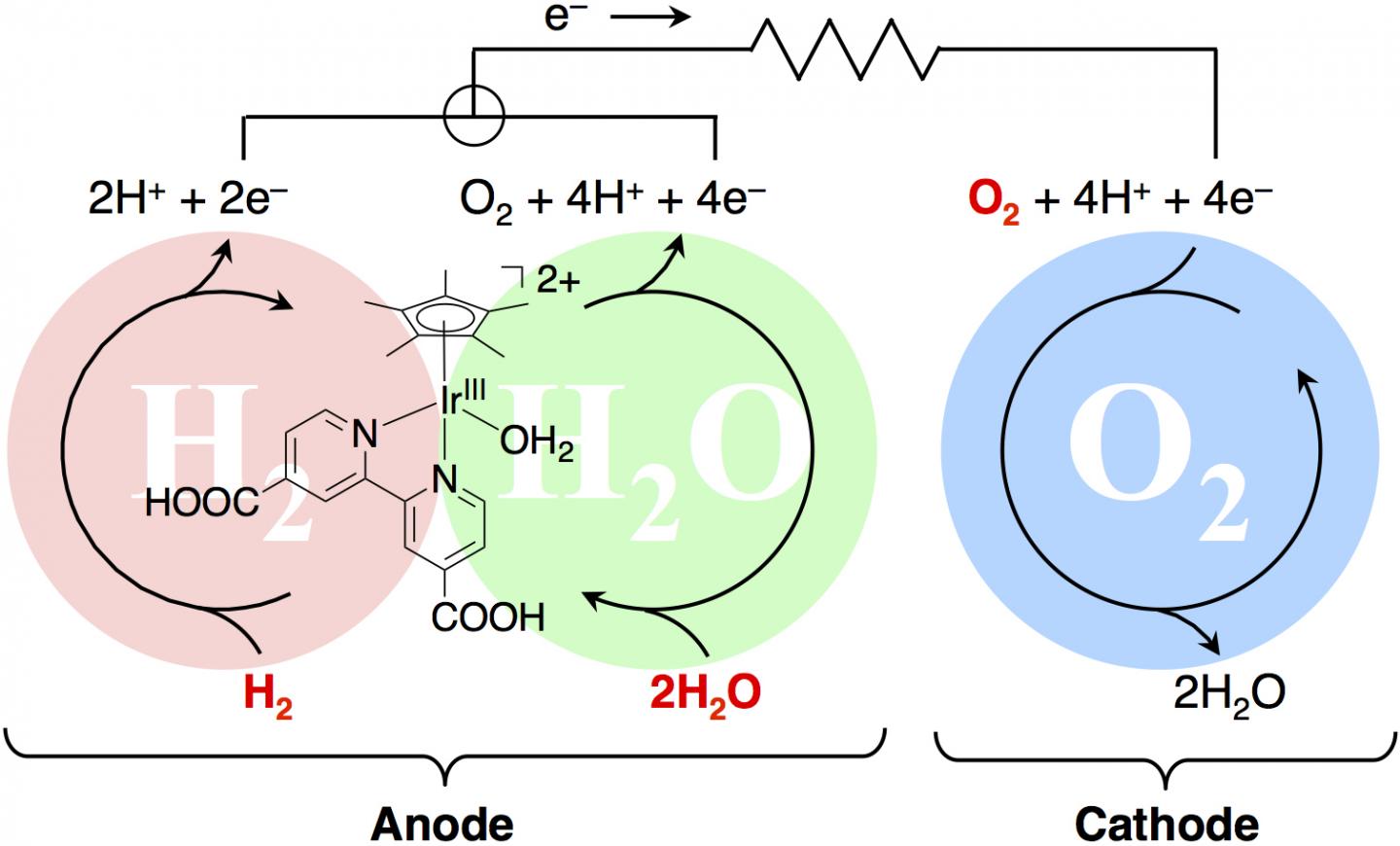
“People have tried before to replicate the behavior of hydrogenase and photosystem II artificially, but ours is the first study to combine these two very specific biological functions into a single catalytic system that can do both.” Hydrogenase is an enzyme which is found in organisms that consume hydrogen for energy, kind of like a natural fuel cell. Photosystem II allows plants to use sunlight to turn water into oxygen. Both processes involve an element of oxidation.
Researchers used the metal iridium in which to synthesize a catalyst capable of taking in and releasing several electrons. They then demonstrated how their catalyst produced electrical power in a fuel cell by accepting electrons from hydrogen. Once the chemical had been isolated, x-ray diffraction was used to gain a deeper understanding as to its structure and behavior. “The power output of our system is still rather low for any practical applications, but this work represents a unique demonstration of two different kinds of energy generating processes from a single catalyst,” confirmed the corresponding author Professor Seiji Ogo. “We hope these findings will show that chemists still have much to learn from natural processes.”
More News to Read
- Help Comes in the Form of Biosensors for Cystic Fibrosis Patients
- Researchers Use Gold to Improve Microlasers
- Researchers Get a Much Closer Look at Exoplanets Thanks to New Telescope Attachment
- New Hubble Imagery Captures Strange Warping Caused By A Past Cosmic Collision
- Smart Medical Tattoos That Change Color When a Problem is Detected

