Researchers from Harvard’s Wyss Institute for Biologically Inspired Engineering, Brigham and Women’s Hospital the Mayo Clinic and MIT have come together in a study to demonstrate how a new class of hydrogel-based embolic agents could help to stop excessive bleeding, even in patients with bleeding disorders or on blood-thinners. This eliminates most of the problems associated with current methods. Having published the study in Science Translational Medicine, the researchers clearly demonstrate how hydrogel can be effectively delivered by catheters and injected into blood vessels to build ample blockages.
“This new approach to vascular embolization is based on a hydrogel composite with phase properties we can reliably control with mechanical pressure. It completely blocks vessels in situations where other methods fail such as in vascular areas that are highly convoluted or subject to unusual blood pressures, and importantly, it still works when normal blood coagulation is impaired like in patients receiving blood-thinners or suffering from an intrinsic inability to efficiently form blood clots,” stated Ali Khademhosseini, Ph.D., an Associate Faculty member of the Wyss Institute and a Professor at the Harvard-MIT’s Division of Health Sciences and Technology and Brigham and Women’s Hospital.
Back in 2014, Khademhiosseini and another author on the current study, Bradley Olsen Ph.D., an Associate Professor of Chemical Engineering at MIT, introduced Shear-Thinning Biomaterial (STB) to the world and showed how if applied to large wounds, it can seal them off and stop any bleeding. STB is a malleable hydrogel that consists of gelatin molecules combined with silicate nanoplatelets. When this mixture is put under pressure (i.e. through the pushing of a syringe) it flows or ‘thins’, then when the pressure is relieved, it solidifies again, this time creating a tight barrier as it does.
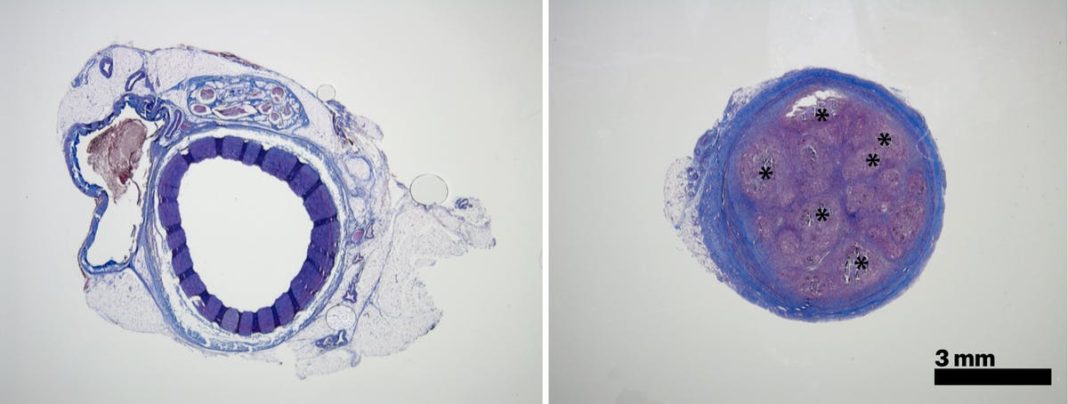
The focus of the present study is on investigating the possibility of using standard catheters in which to deliver STB to hard to reach places within the body in an order to block blood flow where needed. After optimizing the formulation of the STB, the researchers proceeded to use the formula in studies involving mice and pigs.
“In the animals, we saw that STB’s delivered with standard clinical catheters into centrally located vessels, formed very effective casts, without leaking or fragmenting, excluding any risk of pulmonary embolisms down the line” confirmed Reginald Avery, first author of the study. “Moreover, the induced embolization was biodegraded and remodeled into a more natural tissue by infiltrating cells over time.” Moving forward the team are hopeful that STB’s could also be coupled to specific stimuli that attract extra immune cells and enable a faster remodeling of the clot.
More News to Read
- Are Enzymes the Answer to Curing Diseases Such as Cancer and Diabetes?
- NASA’s First 3D Printed Rocket Part is Ready for Testing
- New Breakthrough, Now Cells Can Be Programmed to Fight Disease
- Researchers Discover Bacteria Can Damper the Effects of Chemotherapy
- Physicists Decipher Magnetic Ordering in New MultiFerroic Material