A group of researchers from Indiana University has found a way that could revolutionize the long-term storage possibilities for nuclear waste. In the recent study, the scientists demonstrate how a new chemical principle has the potential to significantly reduce the number of harmful radioactive byproducts that make up nuclear waste by carefully extracting them from it. The same method could also be used in molecules to extract toxic chemicals from soil and water.
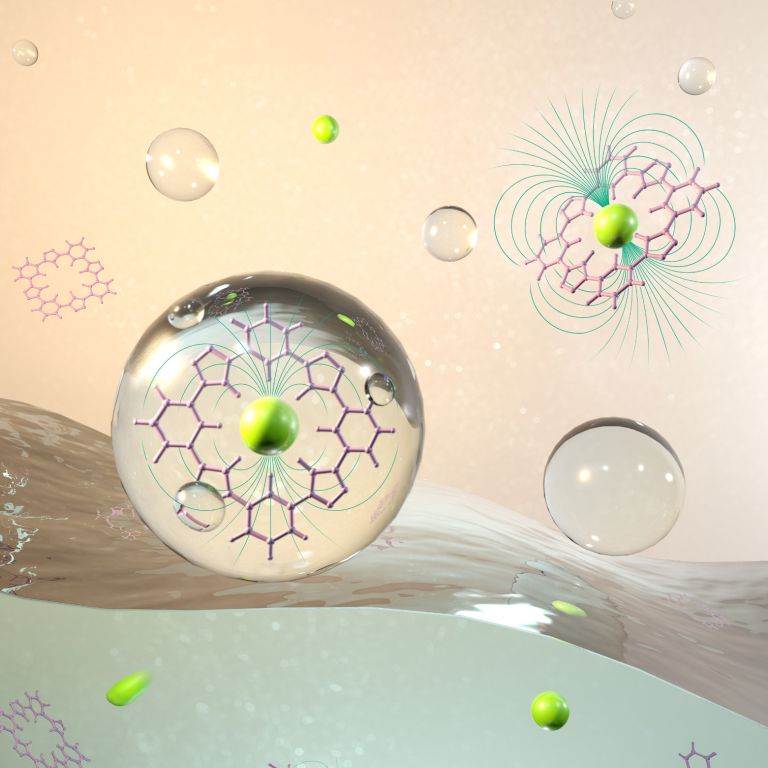
“This work represents a major step forward in the effort to engineer specially designed nanostructures by providing a new, highly accurate method to predict how these molecules will behave in solution,” said Amar Flood, lead author on the study and professor in the IU Bloomington College if Arts and Sciences’ Department of Chemistry. The work carried out by Flood demonstrates just how difficult it is to effectively predict how an engineered molecule will perform once made. This is mainly due to the fact current methods to create molecules are only designed to work in isolation. Yet molecules exist in solutions.
Flood compared this situation to that of creating a machine in outer solace then putting it at the bottom of the ocean. It’s not going to work the same as it would have originally. This is a problem because creating artificial molecules to carry out a specific task is no easy feat. However, in Flood’s lab, a special molecule was developed called a cyanostar in which to help with this issue. The cyanostar is a five-sided star-shaped object made up of carbon and nitrogen atoms. The empty center is the so-called “lock” which causes negatively charged molecules the key to attach to the center and break away from their original host. If the solution in some way warps the lock or fills up, there’s a strong possibility the key will no longer work.
As well as helping to reduce nuclear waste, this kind of technology could also be used to extract chloride from water, collect lithium ions used in renewable power, or extract excess harmful chemical fertilizers from the soil. Using the methods demonstrated in the study, Flood is confident chemists can now begin to design new molecular reactions. To test their own theory, Flood and colleagues took a sample of triazolophane and applied their newly developed method to it. Mixing the molecule with common chemical solvents used to remove unwanted ions from liquids, the team successfully predicted the effectiveness of the molecules.
“The current paradigm only works for molecular designs on the drawing board, in theory,” confirmed Yun Liu, the primary researcher responsible for this method and a Ph.D. student in Flood’s lab. “But we want to make molecules that will work in practice to help solve problems in the real world.”
More News to Read
- Researchers Discover Ground Ice on the protoplanet Vesta
- Scientists Uncover Evidence That Suggests Mars’ Crust Isn’t As We Thought
- Researchers Have Developed a Prototype Smart Pill to Diagnose and Treat Diseases
- Move Across Even the Deepest of Snow With Ease With Drift Boards
- Keep Safe and Be Seen With the Next Generation Million Mile Light

