Progeria is a rare genetic condition that causes a child to age prematurely and affects roughly 1 in every 4 million newborns across the world. The average lifespan of children that suffer from progeria is just 14. However, researchers at the Salk Institute have been studying the disease and have uncovered a process that happens in progeria that could prolong the lives of sufferers and others.
People with progeria appear to have an overactive protein synthesis going on which leads scientists to believe that if we can reduce that synthesis, we’ll be able to extend the person’s lifespan. This could help not just those suffering from progeria, but for healthy people too to allow them to live a longer life. Martin Hertzer is vice president and chief science officer of the Salk Institute and senior author of the paper. He says, “The production of proteins is an extremely energy-intensive process for cells. When a cell devotes valuable resources to producing protein, other important functions may be neglected. Our work suggests that one driver of both abnormal and normal aging could be accelerated protein turnover.”
In regards to Hutchinson-Gilford progeria, the mutation occurs in a protein that’s found in the cell nucleus, called lamin A. However, as of yet, it’s unclear how this single mutation causes the intense aging symptoms of the disease. What scientists do know is that it’s not just lamin A that’s affected in progeria. “We analyzed all the proteins of the nucleus and instead of seeing rapid turnover in just mutant lamin A and maybe a few proteins associated with it, we saw a really broad shift in overall protein stability in the progeria cells,” said Abigail Buchwalter, Salk Staff Scientist and first author of the paper.
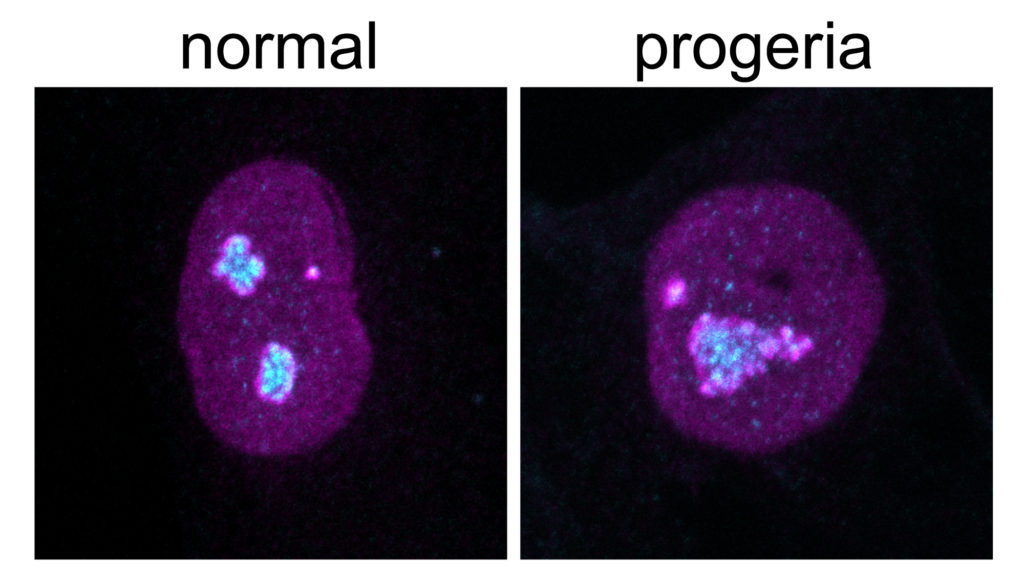
Buchwalter said herself that this change in protein metabolism wasn’t one they were expecting. As well as the fast turnover of proteins, the nucleolus was much larger in the prematurely aging cells in comparison with those that aged normally. The team also discovered that in the healthy cells the nucleolus size grew as they aged. This suggests that size could be a good indication of the nucleolus’ age and could potentially be used in targeting therapies to counteract both normal and premature aging.
Hertzer and colleagues intend to carry on studying how nucleolus’ size can act as a reliable biomarker for aging. “A biomarker such as this that tracks aging would be very useful, and could open up new ways of studying and understanding aging in humans, ” he says.
Article Via Salk Institute / Study Published @Nature Communications
More News to Read
- Do Physicists Need to Change the Way They Measure Quantum States?
- Researchers Discover Response in Liver Could Actually Help Treat Alcoholism
- Ancient Nova Explosion Recovered by 580-year-old Korean Recording
- Scientists Use VLA to Measure Galaxy’s Magnetic Field Billions of Light-Years Away
- Who Said Oil and Water Don’t Mix? New Study Suggests Otherwise
