A cell’s cytoplasm consists of thousands of swarming protein that come together and break apart as they go. Through this crowded cytoplasm, organelles such as mitochondria and lysosomes must travel to deliver materials to different parts of the cell. In a recent study, researchers have come to realize that these organelles and other intracellular components will likely experience different environments as they travel through. For example, the nucleus of a cell may feel the cytoplasm as a liquid, honey-like material, whereas the mitochondria may sense it to be more like toothpaste.
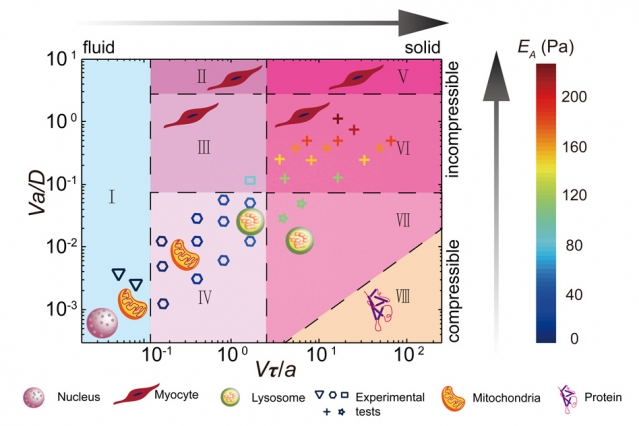
The study, led by Ming Guo, the Brit and Alex D’Arbeloff Career Development Assistant Professor in MIT’s Department of Mechanical Engineering, revealed that an organelle feels a certain resistance in the cytoplasm and the strength of the resistance depending on the size and speed of the organelle. Some organelles have to work harder than others at moving through a cell’s cytoplasm and will, therefore, feel more resistance. To give people an idea, Guo and colleagues drew up a phase diagram to show the type of material the cytoplasm would resemble depending on the size and speed of the organelle, through the perspective of the organelle.
“With this phase diagram, as long as you tell me the size and speed at which an organelle moves, I can tell you what mechanical environment it sees,” says Guo. “A drug with a 100-nanometer diameter will feel a very different resistance than something that is 500 nanometers wide. This can be a guide to understanding how a drug is delivered and transported inside a cell.” While most other studies involving the transport of materials within a cell have concentrated on the drivers of that transport, Guo and team focused on the resistance of the surrounding material instead.
One of the things Guo wanted to test was whether or not the surrounding cytoplasm in living cells would crowd major organelles such as mitochondria and lysosomes. To do this, the team experimented on living mammalian cells into which they injected small plastic beads. They then used tiny tweezers in which to drag the bead across the cell at a constant speed, while they measured the resistance. The group figured that a cytoplasm’s resistance stems from poroelasticity and viscoelasticity and the more poroelastic cytoplasm is the harder it is for an organelle to push water. On the opposite side, viscoelasticity is to do with how fast a cytoskeleton can change the configuration. The faster it can do this, the more fluid like it is. So, researchers figured that an organelle would feel less resistance in this environment.
After analyzing the results from the study, Guo and his colleagues discovered that the amount of resistance felt was directly related to the bead’s size and speed as it moved across the cell. The results were that the larger the bead, the more resistance there was. They also discovered, after plotting their findings on a diagram, that the organelles do in fact experience a range of resistances while inside the cytoplasm. “If you ask a nucleus, they would tell you the cytoplasm is like honey, because they are really large and slow, and they don’t feel cytoskeletal structures – they only feel the viscous disassembled protein solution, and have very small resistance,” says Guo. “But mitochondrial would say it’s like toothpaste, because they are smaller and faster, and are sometimes blocked by these constantly changing structures. So their views are limited by their own size and speed.”
More News to Read