Biomarker molecules are indicative of disease states and physicists at the University of Pennsylvania have just created a new graphene-based sensor that may soon be used as a low-cost way to test for them. The research was a collaboration with Penn chemists, and was led by Jinglei Ping, a postdoctoral researcher in the Department of Physics & Astronomy in Penn’s School of Arts & Sciences, A.T. Charlie Johnson, a physics professor, Katherine Pulsipher, a post doctorate in the Department of Chemical and Biomolecular Engineering in the School of Engineering and Applied Science.
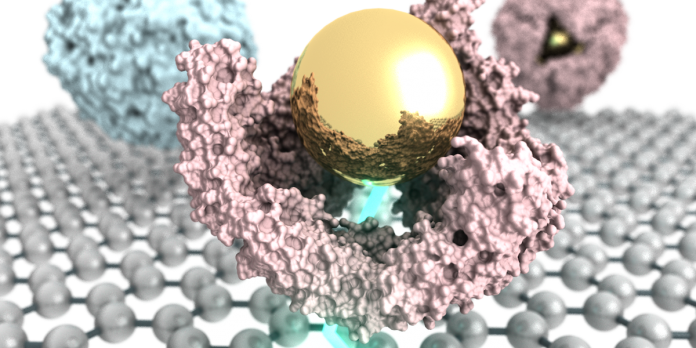
The graphene micro electrode is a sensor that measures the current flowing between a liquid sample and the surface of the graphene. Chemistry professor Ivan Dmochowski and his group have been studying how inorganic nanoparticles interact with proteins, specifically, a protein called ferritin. This particular protein is known for storing large amounts of iron. One of thermophilic ferritin’s features is that it can assemble in high salt and disassemble in low salt. Dmochowski explained, “What we found some years ago, is that we could take salt out of the equation and work in low salt where the protein would normally be dissembled and put in a gold nanoparticle with a coating that makes it very charged in solution with the ferritin. The nanoparticle is spontaneously encapsulated by the protein, like a ship in a bottle.”
Nanoparticles which are inorganic are largely considered to be the next big thing when it comes to diagnostic and therapeutic agents. One of the unusual features of ferritin is that it has four triangles shaped, nano-sized pores. The team wanted to know whether these pores are maintained as the protein assembles around the gold nanoparticle and so they decided to try and use the new graphene sensor to find the answer. “We were trying to think about something different that we could do with our sensor,” explains Johnson, “and we came up with the idea that we could use it to learn something about this bio-nanosystem.”
During their research, the team created a pore free molecule and compared it to the wild types with pores that exist in nature. What they discovered was that the wild types the current were flowing via the metal particle, but when there were no pores, the current was shut off and unable to flow through. The results from the study are promising and Dmochowski said the protein-encapsulated gold nanoparticles used in the study will hopefully be used as building blocks for future uses in fields such as chemistry and medicine. “This research could provide fundamental building blocks that you would then integrate with many of these types of protein-nanoparticle assemblies to make devices,” he says.
Dmochowski says there’s no reason why those same ferritin shells can’t be used instead of nanoparticles to put a therapeutic protein inside and crosslink the protein. “In that scenario, it’s protecting another protein, and then you can functionalize the outside of the ferritin to direct it to places it wouldn’t normally go in the body,” he says. However, finding real-life samples to work with is can be difficult as they tend to be more complicated in themselves. Johnson says, “The sensor works well in solutions that are salty, which is an advantage, and we have another paper that’s been published where we showed that it can work in solutions such as human serum which is, relatively speaking, very complicated.” Dmochowski confirms, “We’ve been studying this for a long time, and if it’s often the case in chemistry you have to come up with new methods of studying new types of matter”.
More News to Read
- Most Accurate Measurements of Dark Matter and Dark Energy Revealed by Dark Energy Survey
- Scientists Suppress Instabilities That Can Damage Fusion Reactors
- Can Twitter Step it Up and Provide Real Time Monitoring During Disaster Situations?
- How to Make a Supercomputer?
- Scientists Use New Model to Better Predict Brown Dwarf Weather Forecasts
