It’s an exciting time for gene editing currently as scientists achieve the first ever safe repair of a single-gene mutation in a human embryo. Using the CRISPR-Cas9 system, medical professionals used the technique to correct the mutation for a heart condition. The procedure was carried out early enough in the embryonic development so that the defect wouldn’t be passed down to further generations.
The study is an important breakthrough and could pave the way for improved in vitro fertilization (IVF) outcomes further down the line as well as finding cures for even a few of the thousands of diseases caused by single gene mutations. Juan Carlos Izpisua Belmonte, a professor in Salk’s Gene Expression Laboratory and corresponding author of the paper commented that, “Gene editing is still in its infancy so even though this preliminary effort was found to be safe and effective, it is crucial that we continue to proceed with the utmost caution, paying the highest attention to ethical considerations.” Although gene editing has become relatively easy to carry out for scientists these days, they do still proceed with much caution. This is partly to avoid introducing any mutations into the germ line.
*Hypertrophic cardiomyopathy (HCM) affects around 1 in every 500 people and is the most common cause of sudden death in otherwise healthy, young athletes. A dominant mutation in the MYBPC3 gene is the cause of it and there’s a 50 percent chance of a carrier passing it to their offspring. If the mutation in the embryo was corrected it would prevent the disease in children as well as their descendants. During the study, researchers produced induced pluripotent stem cells from a sample taken from a male with HCM. They also developed a CRISPR-Cas9 gene editing strategy that was able to target specifically the mutated copy of the MYBPC3 gene for repair. The MYBPC3 gene was cut by the Cas9 enzyme allowing the mutation to be fixed in the next round of cell division. Researchers then used IVF techniques to inject top gene-editing components into healthy donor eggs which had been newly fertilized. They were then able to monitor just how well the mutation was repaired.
This method proved to be both extremely safe and efficient, much to the surprise of the researchers. No strange mutations were induced and a high number of embryonic cells were repaired. They also developed a way in which they could ensure the repair happened consistently in all of the embryonic cells which is a bonus as spotty repairs have been known to cause mutations. “Even though the success rate in patient cells cultured in a dish was low, we saw that the gene correction seems to be very robust in embryos of which one copy of the MYBPC3 gene is mutated,” states Salk staff scientists and one of the paper’s first authors, Jun Wu. “Our technology successfully repairs the disease-causing gene mutation by taking advantage of a DNA repair response unique to early embryos.” However, it is still early days and both Izpisua Belmonte and Wu agree further research is required to ensure no unintended effects occur.
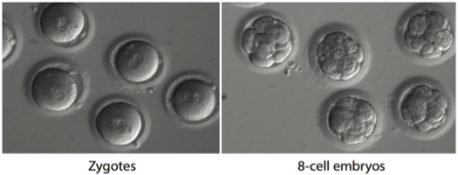
Research Via Salk Institute
*Hypertrophic cardiomyopathy (HCM) is a condition in which the heart muscle becomes thick. Often, only 1 part of the heart is thicker than the other parts. The thickening can make it harder for blood to leave the heart, forcing the heart to work harder to pump blood. It also can make it harder for the heart to relax and fill with blood. Via MedLine Plus
More News to Read
- Want High-Quality Video but Sick of Buffering? Then Look No Further
- First Modeling of Plasma Turbulence in Tokamaks Studies Recycled Atoms’ Effect on Fusion
- Latest ESA Research Leads to Better Cameras for Precision Agriculture
- This Affordable Lead Sensor Could Keep an Eye On Your Water Quality
- Meet Root – The Robot That Will Teach You Coding in No Time!