For almost a decade perovskite based solar cells have been improving in efficiency, increasing from four percent to 20 percent between 2009 and 2017, and is expected to outpace the more familiar silicon based cells, according to many experts. However, despite the better performance and improved devices, researchers are unsure of the reasons behind perovskites efficiency.
Now, in a study published in Science Advances, scientists from Stanford University and the Department of Energy’s SLAC National Accelerator Laboratory reveal how they’ve come to understand the material after filming the behaviors of atoms on an electron camera.
Solar cells work when the energy from light dislodges the material’s electrons, which then move to the other side of the cell making it negatively charged and leaving their original location positively charged. The difference between the charges creates a voltage that powers the devices to which the cell is connected.
The capability to do this well is dependent on the structure of the components of the cell and how much or little it interferes with the electrons ability to move uninhibited through the solar cell. For cells made of silicon, which typically has a very ordered structure, this means the particles must be perfectly aligned and 100 percent free of defects, a result achieved in a laborious production process, slowing down the time it takes and increasing manufacturing costs.
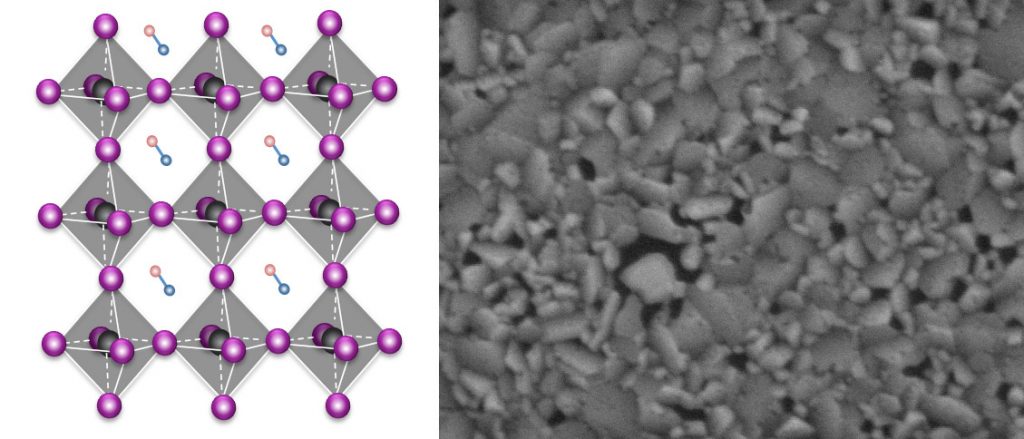
On the other hand, perovskites are made via a chemical solution and left to reduce to a film substance. The process is simpler overall and quicker which helps lower prices and makes perovskite cells popular. Additionally, the resulting film is flexible and light, making it adaptable to many types of uses.
But to learn more about the way perovskites work in solar cells, Xiaoxi Wu of Stanford’s Institute of Materials and Energy (SIMES) and her colleagues studied a material composed of lead, iodine, and methylammonium, whose atomic octahedra structure is similar to those in many perovskites.
The research team created a kind of stop motion movie by exposing the extremely thin film to a very fast laser pulse and measuring the electron reactions in a process known as ultrafast electron diffraction, or UED.
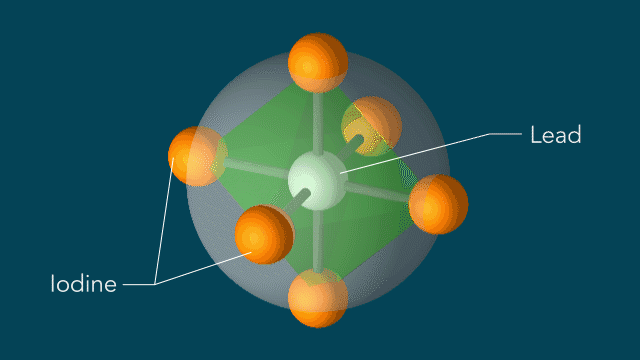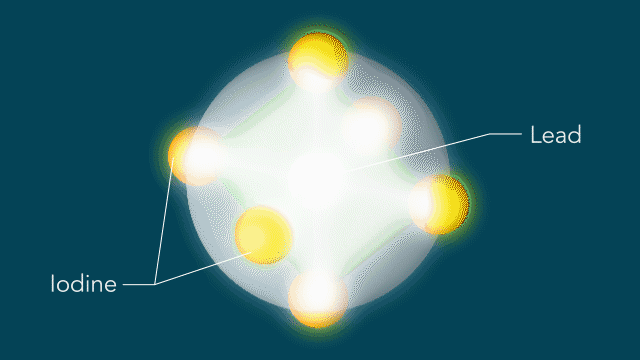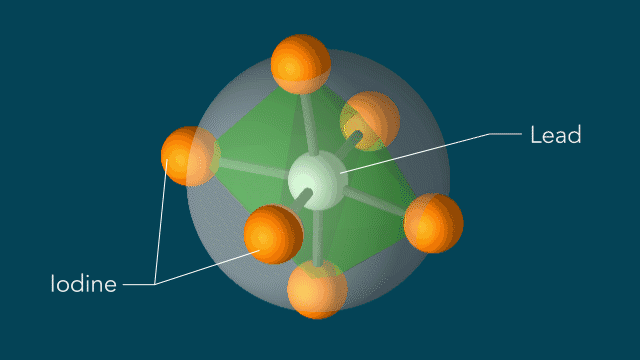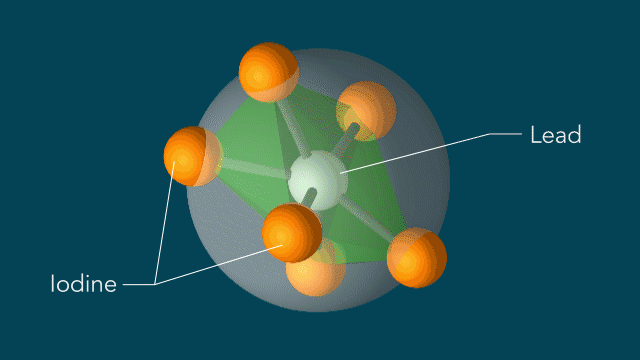
Instead of the light causing the atoms to move equally in the regular shapes, Wu and team found that the light changed the movement of the iodine atoms, in turn distorting the shape of the center lead atom. According to Wu, these altered shapes and movements could be the key to perovskites’ efficiency by allowing a charge to move through the cells uninhibited and without getting ensnared in the material.
With more study, these insights could lead to better perovskite solar cells production for even more efficient charging.
More News to Read
- Why Does Adobe Flash Need to Die?
- Breaking Ground in Neutrino Physics Research with the Biggest Neutrino Detector yet
- UM’s New Nanoparticle Research Could Lead to Cloaking Technology
- Does Physical Activity Affect the Brain’s Aging Process?
- Gaining a Deeper Understanding of the Relationship Between DNA and Cell Function

