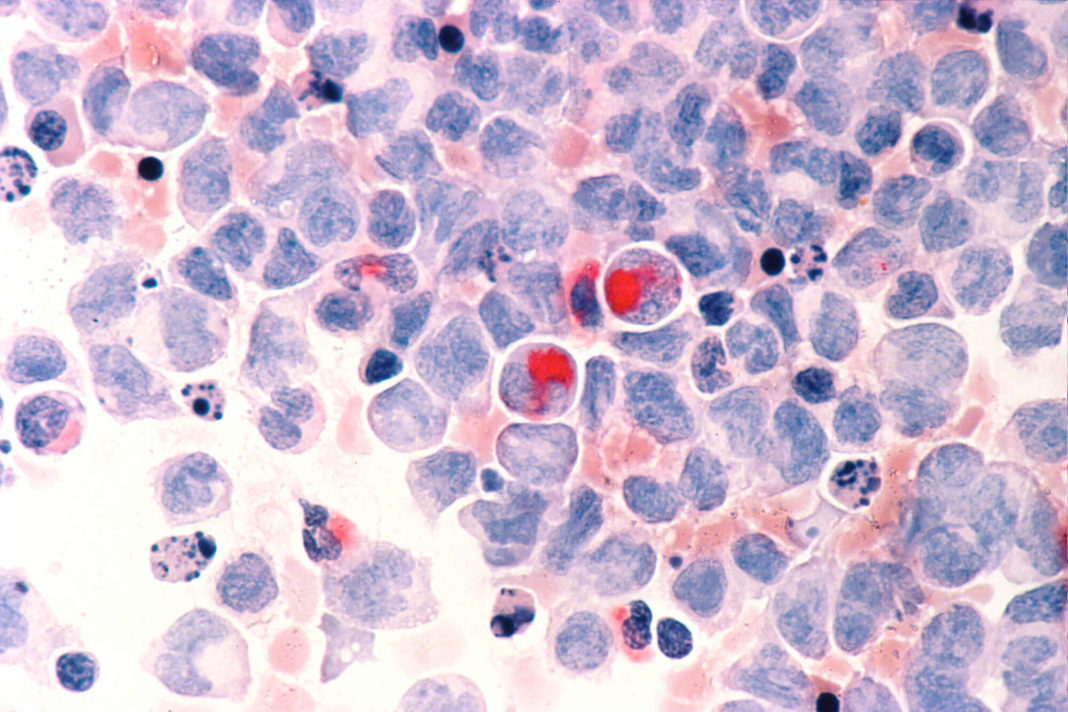
A new immunotherapy drug was given to a 73-year-old patient last year. He seemed the perfect candidate to receive the new drug, *Atezolizumab, or Tecentriq, but things just didn’t go to plan. The patient started the course of treatment in June, and within six weeks was removed from the program. He sadly died just two months later.
This isn’t the first case of this nature either. Several other patients have experienced similar situations where not only has the treatment failed to treat cancer, but it’s accelerated the disease and made the tumors grow faster. Six such cases had been found out of a total of 155 studied.
“There’s some phenomenon here that seems to be true, and I think we cannot just give this therapy randomly to the patient,” says the author of the study and oncologist at UC San Diego, Dr. Shumei Kato. “We need to select who’s going to be on it.” But, not everyone is as convinced. Dr. Vinay Prasad is an assistant professor in the Division of Hematology-Oncology at Oregon Health and Science University, and he says, “Tumor growth is not a precise measurement, and if you measure lots of people, some will have faster growth just because of the error in the test. At the end of the day, I think the main point is if you use drugs where “randomized control trials” show benefit, you will be good. But, given that immunotherapies are usually approved based on early findings, he added, “don’t be surprised if you harm patients.”
Patients and doctors should not be deterred by these results and should still consider immunotherapy a viable option for some cases. In recent studies, scientists found that all of those who suffered from the hyperprogression of tumors had amplifications in the MDM2 gene family. Moving forward, with thanks to the study, doctors are hoping to be able to better identify at-risk patients before considering immunotherapy as a treatment option. One such way of dong this would be by submitting tumors for genetic testing first.
The problem is that some patients who receive immunotherapy treatments can show signs of a “pseudo-progression.” This is where the tumor appears enlarged on the scan, but in reality is simply where swarms of immune cells NIL attacking cancer. But, for reasons unknown, immunotherapy doesn’t work on most patients and even when it does, the side effects, although rare, can be life-threatening. Some reported cases even include patients suffering from a rapid onset of diabetes after receiving immunotherapy treatment. But at least the cancer was being attacked at the same time in those cases. It is still early days regarding offering this type of treatment and new developments are being made all the time. But for now, all we can do for now is continue as we are, and keep improving as we go along.
*Atezolizumab: (trade name Tecentriq) is a fully humanized, engineered monoclonal antibody of IgG1 isotype against the protein programmed cell death ligand 1 (PD-L1). It is currently in clinical trials as an immunotherapy for several types of solid tumors.[1] It is under investigation by Genentech / Roche.
Related Links;
- Hyper-progressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate / Clinical Cancer Research
- Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy / NCBI
- Cancer researchers worry immunotherapy may hasten growth of tumors in some patients / StatNews
More News to Read