A new and exciting type of gene therapy treatment has been implemented as so far its results have been incredible. It works by altering the patient’s own supply of blood cells into cancer-fighting cells and so far out of those who took part in the initial trials, a third of those suffering from advanced lymphoma are showing no signs of it six months later after having just one dose of the treatment.
In total, the study that was sponsored by Kite Pharma resulted in 36 percent of those taking part going into complete remission after receiving the therapy. This is a fantastic result and could provide a new alternative to treating cancer where others have failed miserably. “These results with axicabtagene ciloleucel are exceptional and suggest that more than a third of patients with refractory aggressive NHL could potentially be cured after a single infusion of axicabtagene ciloleucel,” said Jeff Wiezorek, M.D., Senior Vice President of Clinical Development at Kite Pharma.
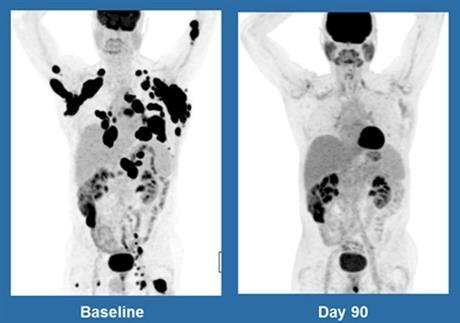
Like with any treatment, there is an element of risk involved with this new type of gene therapy which is to be expected when you’re altering a person’s cells to behave in a way that’s unconventional. But, the ratio is low. Out of the 101 people that participated in the study, only two are said to have died from the actual treatment, not cancer. And, with a 41 percent success rate in having at least shrunk cancer by half, that’s pretty good going.
The way the treatment works is by filtering the patient’s blood to extract T-Cells from the immune system. These are then modified in the lab, and a gene is then added that forces them to become cancer targeting cells. Finally, the blood is pumped back into the patient where the altered cells get to work multiplying and attacking cancer. Dr. Fred Locke is a blood expert at Moffitt Cancer Center in Florida, and he says, “The numbers are fantastic, these are heavily treated patients who have no other options.” Moving forward, the only thing left to do now is getting it approved as an official form of treatment by the FDA and Europe, which should happen by the end of the year at the latest.
More News To Read
- For the First Time Ever Researchers Experimentally Achieve a State of Supersolidity
- Stem Cells Used to Create Artificial Embryo for the First Time Ever
- Can You Imagine How the World’s First Spaceport Will Look Like?
- Scientists Announce the Discovery of 7 New Habitable Planets That Aren’t Actually Habitable After…
- Movies and Games Become One with this Crazy New Technology